Neurexin-3 subsynaptic densities are spatially distinct from Neurexin-1 and essential for excitatory synapse nanoscale organization in the hippocampus
Por um escritor misterioso
Descrição

PDF] Modeling a Neurexin-3α Human Mutation in Mouse Neurons Identifies a Novel Role in the Regulation of Transsynaptic Signaling and Neurotransmitter Release at Excitatory Synapses

Nanoscale synapse organization and dysfunction in neurodevelopmental disorders - ScienceDirect
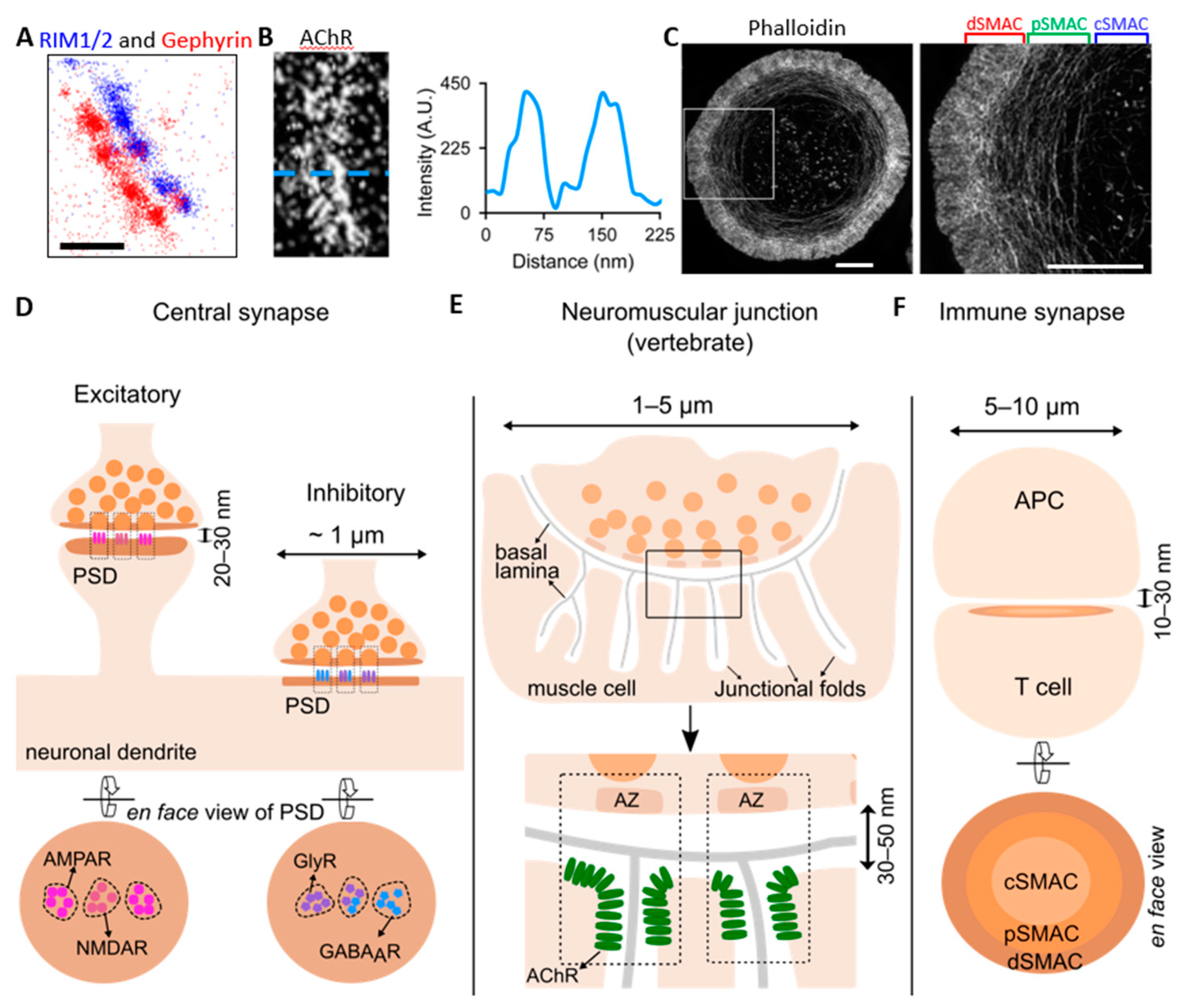
Membranes, Free Full-Text

Nrxn3α alternative splicing regulates inhibitory synapses in

3D-SIM of Inhibitory Synapses (A) Schematic of the inhibitory synapse
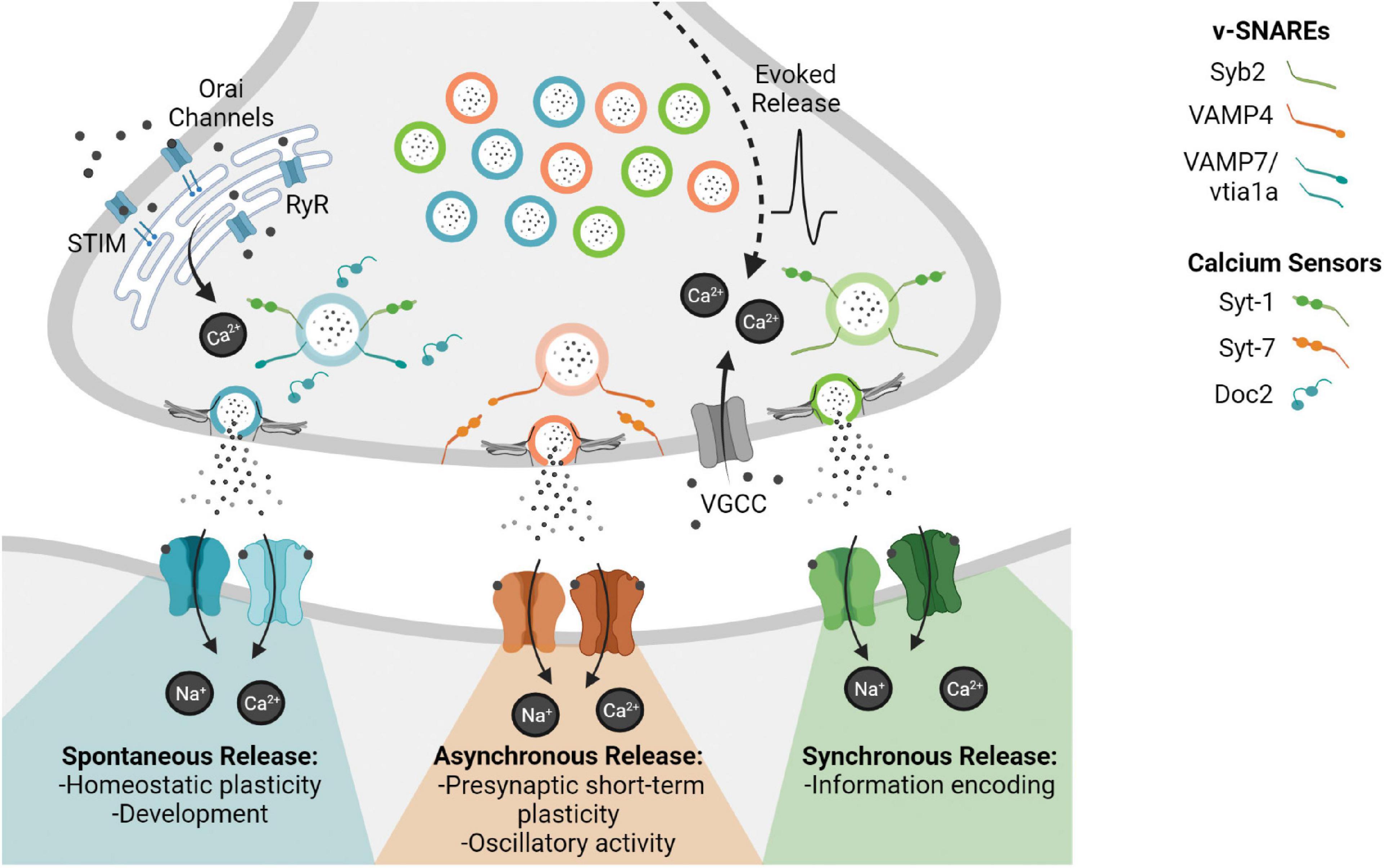
Frontiers Nano-Organization at the Synapse: Segregation of Distinct Forms of Neurotransmission

Synaptic Neurexin Complexes: A Molecular Code for the Logic of Neural Circuits. - Abstract - Europe PMC

Contribution of Postsynaptic Molecules to AMPA Receptor Nanodomain Organization
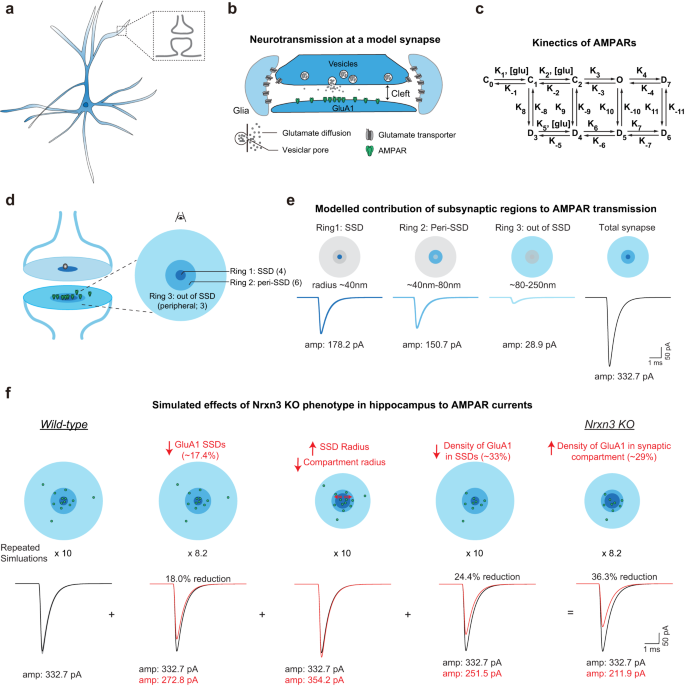
Neurexin-3 subsynaptic densities are spatially distinct from Neurexin-1 and essential for excitatory synapse nanoscale organization in the hippocampus
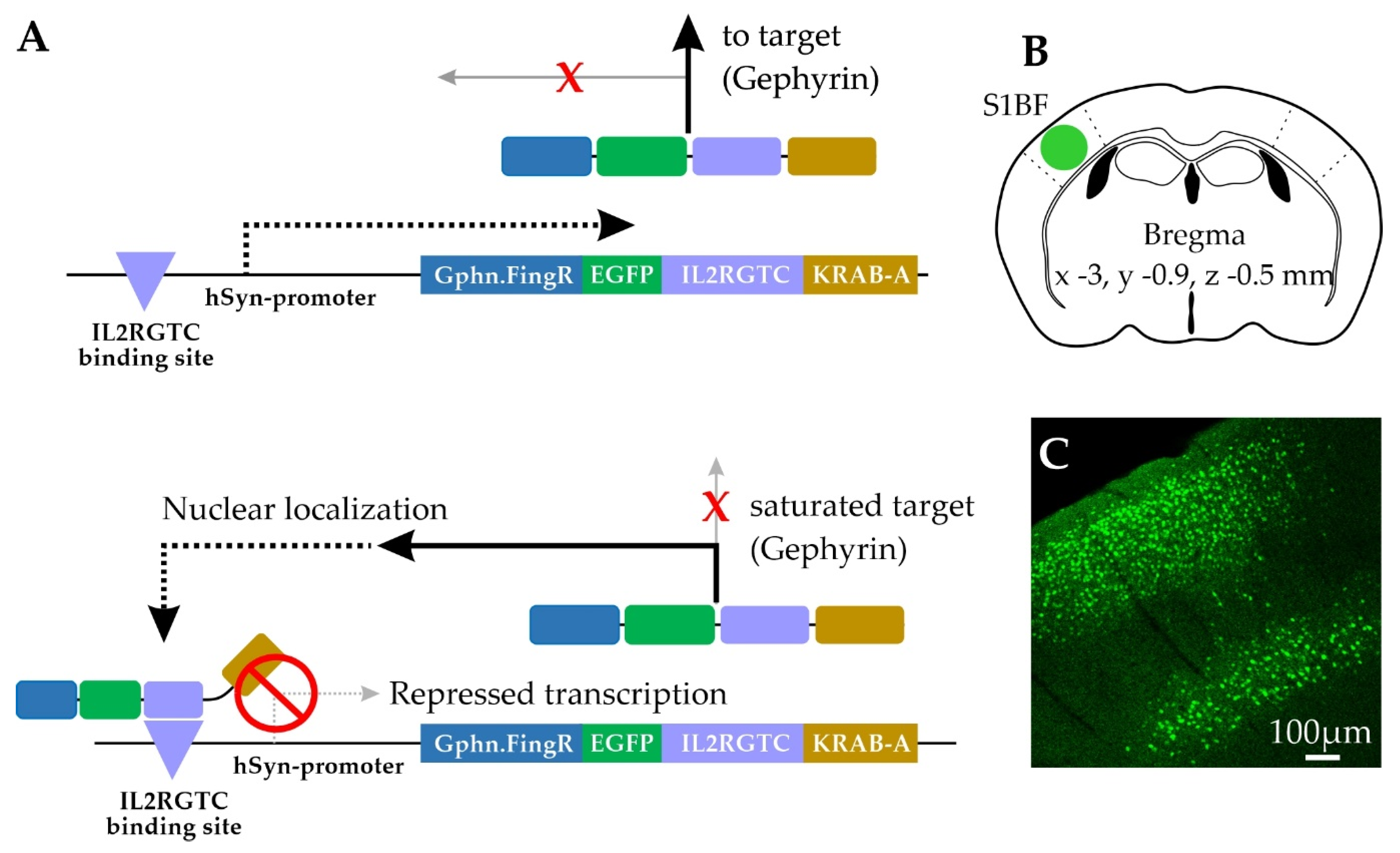
IJMS, Free Full-Text

Molecular replacement with Nrxn3 A687T SS4 enhances presynaptic release
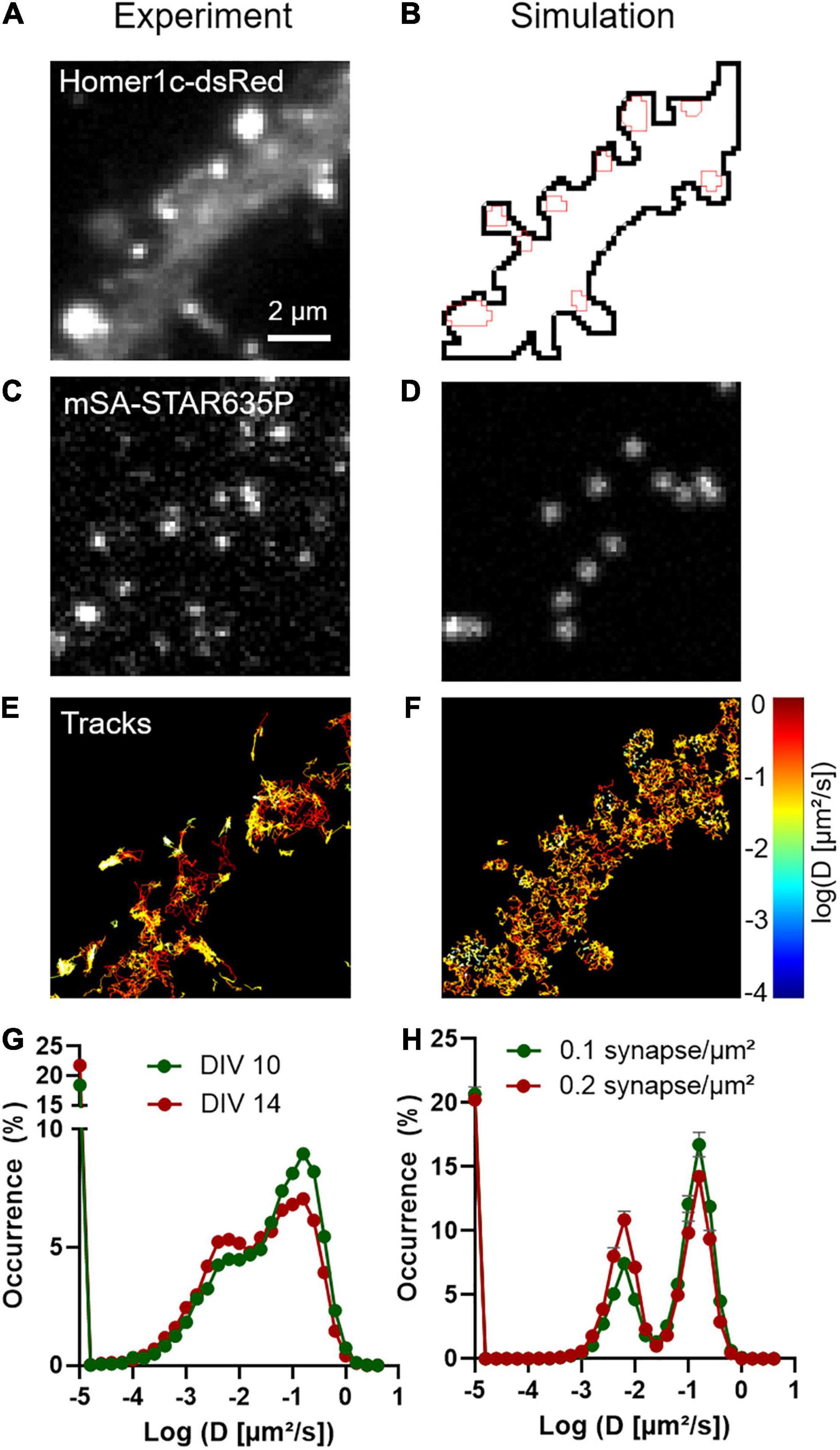
Frontiers High-Resolution Fluorescence Imaging Combined With Computer Simulations to Quantitate Surface Dynamics and Nanoscale Organization of Neuroligin-1 at Synapses

Modeling a Neurexin-3α Human Mutation in Mouse Neurons Identifies a Novel Role in the Regulation of Transsynaptic Signaling and Neurotransmitter Release at Excitatory Synapses

Neurexin-2 restricts synapse numbers and restrains the presynaptic release probability by an alternative splicing-dependent mechanism

Subsynaptic positioning of AMPARs by LRRTM2 controls synaptic strength
de
por adulto (o preço varia de acordo com o tamanho do grupo)